When designing footprints for electrolytic capacitors it is important to place clear indicating marks to show the components’ orientation. Since this style of capacitor is polarized (they have to be placed in a certain orientation) they must have indicating marks on the PCB to help determine how they should be placed. Clarity in marking components is key to making sure that the manufacturing of your design goes smoothly and the blue smoke does not escape your capacitors. Even more so are electrolytic capacitors made from tantalum as they tend to have catastrophic consequences when they are powered up backwards.
So for identification, led’s comes with a unique way to identify its terminals as Anode or Cathode. Sometime the diode symbol creates confusion too.Identifing a LED's the cathode and anode of a led is very easy by looking inside. LED's or Light Emitting Diode's don’t come with any labeling on it to identify Cathode (-ve,GND) or Anode (+ve). A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic CCD for Cathode Current Departs. A conventional current describes the direction in which positive charges move.
The Electrolytic Capacitor
Electrolytic Capacitors are one of the most popular types of capacitors used on board design. They are low cost and provide a good balance of physical size and capacity. There are four physical flavors of electrolytic capacitors; SMT Can, SMT Case, PTH Radial, and PTH Axial. Each style is marked slightly differently. They are usually marked with a band on the cathode side of the capacitor indicating the negative terminal but there are some exceptions. This is different from the typical schematic symbol which is positive or anode marked!
Schematic Symbol
The typical polarized capacitor will look like the image below on the schematic. The positive or anode side of the capacitor is marked with a “+” symbol. Since electrolytic capacitors are polarized, I use a symbol (shown below) on my schematics.
Schematic symbol for polarized capacitors as shown in Eagle.
SMT Can Style Electrolytic Capacitor
These capacitors are marked on the top of the can with a black mark. The mark’s color sometimes depends on the manufacture however. The plastic base of the capacitor are also chamfered on the positive or anode side.
SMT Can Electrolytic Capactor: Marking indicates negative or cathode side.
Footprint of a typical SMT can electrolytic capacitor.
SMT Case Electrolytic Capacitor
Capacitors of this type usually have tantalum or niobium inside but there are a few polymer electrolytic. Case style means it is shaped similar to a 0805 resistor or ceramic capacitor. Unlike the other packages for capacitors, these are typically positive or anode marked.
SMT case style electrolytics are usually anode/positive marked. Watch out!
Cathode Positive Or Negative
Footprint for SMT case style electrolytic capacitors.
PTH Radial Electrolytic Capacitor
Radial caps have both the anode and cathode leaving one side of the capacitor. 99% of the time these are marked with a contrasting strap down the cathode or negative side of the capacitor.
PTH radial polarized electrolytic capacitor markings.
Footprint for PTH radial style electrolytic capacitors.
PTH Axial Electrolytic Capacitor
Axial style capacitors are not used very often but are interesting in how they are marked. A negative or cathode band runs down the side of them similarly to the radial style but there is an arrow in the marking that indicates which side is negative or the cathode.
PTH axial style electrolytic. The cathode strip points towards the cathode.
Footprint for a PTH axial style electrolytic capacitor.
Next time on the footprint files…
The most important thing to remember is to check your parts data sheet and see how the polarity is marked on the part. Copying how the part looks on your boards silkscreenwill guarantee a much higher success during assembly of the board. I hope this will improve your footprints on your board and make your products and prototypes easier to build. Next time on the footprint files we will be discussing tantalum capacitors.
Check out the previous post in this series: The Footprint Files – Diodes
Was this post helpful? Are there other topics you’d like us to discuss? If so, let us know on Twitter.
Get started today.
Cathode Ray
Learning Objective

- Define a cathode ray
Key Points
- Electrons accelerated to high velocities travel in straight lines through an empty cathode ray tube and strike the glass wall of the tube, causing excited atoms to fluoresce or glow.
- Researchers realized that something was traveling from the anode when objects placed in the tube in front of it could cast a shadow on the glowing wall. Cathode rays carry electronic currents through the tube. Electrons were first discovered as the constituents of cathode rays.
- J.J. Thomson used the cathode ray tube to determine that atoms had small negatively charged particles inside of them, which he called “electrons.”
Terms
- crookes tubeAn early experimental electrical discharge tube, invented by English physicist William Crookes and others around 1869-1875, in which cathode rays, streams of electrons, were discovered
- cathode raysStreams of electrons observed in vacuum tubes
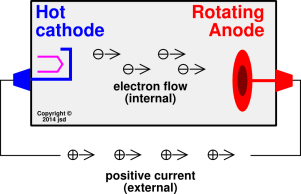
Cathode Rays
Cathode rays (also called an electron beam or an e-beam) are streams of electrons observed in vacuum tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, the glass opposite the negative electrode is observed to glow from electrons emitted from the cathode. Electrons were first discovered as the constituents of cathode rays. The image in a classic television set is created by focused beam of electrons deflected by electric or magnetic fields in cathode ray tubes (CRTs).
Cathode rays are so named because they are emitted by the negative electrode, or cathode, in a vacuum tube. To release electrons into the tube, they must first be detached from the atoms of the cathode. The early cold cathode vacuum tubes, called Crookes tubes, used a high electrical potential between the anode and the cathode to ionize the residual gas in the tube. The electric field accelerated the ions and the ions released electrons when they collided with the cathode.
Modern vacuum tubes use thermionic emission, in which the cathode is made of a thin wire filament that is heated by a separate electric current passing through it. The increased random heat motion of the filament atoms knocks electrons out of the atoms at the surface of the filament and into the evacuated space of the tube. Since the electrons have a negative charge, they are repelled by the cathode and attracted to the anode. They travel in straight lines through the empty tube. The voltage applied between the electrodes accelerates these low mass particles to high velocities.
Cathode rays are invisible, but their presence was first detected in early vacuum tubes when they struck the glass wall of the tube, exciting the atoms of the glass and causing them to emit light—a glow called fluorescence. Researchers noticed that objects placed in the tube in front of the cathode could cast a shadow on the glowing wall, and realized that something must be traveling in straight lines from the cathode. After the electrons reach the anode, they travel through the anode wire to the power supply and back to the cathode, so cathode rays carry electric current through the tube.
History of Cathode Rays
In 1838, Michael Faraday passed a current through a rarefied air-filled glass tube and noticed a strange light arc with its beginning at the cathode (negative electrode) and its end almost at the anode (positive electrode).
Crookes Tubes
In the 1870s, British physicist William Crookes and others were able to evacuate rarefied tubes to a pressure below 10−6 atm. These were called Crookes tubes. Faraday had been the first to notice a dark space just in front of the cathode, where there was no luminescence. This came to be called the cathode dark space, Faraday dark space, or Crookes dark space.
Cathode Positive Or Negative
Crookes found that as he pumped more air out of the tubes, the Faraday dark space spread down the tube from the cathode toward the anode, until the tube was totally dark. But at the anode (positive) end of the tube, the glass of the tube itself began to glow. What was happening was that as more air was pumped from the tubes, the electrons could travel farther, on average, before they struck a gas atom. By the time the tube was dark, most of the electrons could travel in straight lines from the cathode to the anode end of the tube without a collision. With no obstructions, these low mass particles were accelerated to high velocities by the voltage between the electrodes. These were the cathode rays. When they reached the anode end of the tube, they were traveling so fast that, although they were attracted to it, they often flew past the anode and struck the back wall of the tube. When they struck atoms in the glass wall, they excited their orbital electrons to higher energy levels, causing them to fluoresce.
Later researchers painted the inside back wall with fluorescent chemicals such as zinc sulfide, to make the glow more visible. Cathode rays themselves are invisible, but this accidental fluorescence allowed researchers to notice that objects in the tube in front of the cathode, such as the anode, cast sharp-edged shadows on the glowing back wall. In 1869, German physicist Johann Hittorf was first to realize that something must be traveling in straight lines from the cathode to cast the shadows. Eugene Goldstein named them cathode rays.
J.J. Thomson’s Experiment
J.J. Thomson studied cathode ray tubes and came up with the idea that the particles in the cathode beams must be negative because they were repelled by negatively charged items (either the cathode or a negatively charged plate in the cathode ray tube) and attracted by positively charged items (either the anode or the positively charged plate in the cathode ray tube). He called these super tiny pieces of the atom, “electrons.” Through his experiments, Thomson disproved Dalton’s atomic theory, because Dalton’s atomic theory stated that atoms were the smallest piece of the matter in the universe and they were indivisible. Clearly, the presence of electrons negated these portions of Dalton’s atomic theory.
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/crookes%20tube
Wikipedia
CC BY-SA 3.0.

Cathode Ray Experiment

http://en.wikipedia.org/wiki/cathode%20rays
Wikipedia
CC BY-SA 3.0.
Cathode Ray
http://en.wikipedia.org/wiki/Cathode_rays
Wikipedia
CC BY-SA 3.0.
