Dalton was an English chemist and teacher who used experimental evidence to form the atomic theory of matter: All elements are composed (made up) of atoms. It is impossible to divide or destroy an atom. All atoms of the same element are alike. The contributions of John Dalton did not come from his original ideas, but he was dedicated to study hypotheses already existing in his time and, through Quantitative experiments, To demonstrate one by one such theories. The 7 main contributions of John Dalton 1- Theory of the atom or atomic. This was his most important contribution to science. Dalton’s atomic theory proposed that all matter was composed of atoms, indivisible and indestructible building blocks. While all atoms of an element were identical, different elements had atoms of differing size and mass. Dalton’s atomic theory also stated that all compounds were composed of combinations of these atoms in defined ratios. John Dalton FRS (/ ˈdɔːltən /; 6 September 1766 – 27 July 1844) was an English chemist, physicist, and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into colour blindness, sometimes referred to as Daltonism in his honour.
- John Dalton Atom Date
- John Dalton Atomic Model
- John Dalton Atom Model Diagram
- John Dalton Atomic Mass
- Influence Of Atomic Theory
Although a schoolteacher, a meteorologist, and an expert on color blindness, John Dalton is best known for his pioneering theory of atomism.
He also developed methods to calculate atomic weights and structures and formulated the law of partial pressures.
Early Life
Dalton (1766–1844) was born into a modest Quaker family in Cumberland, England, and for most of his life—beginning in his village school at the age of 12—earned his living as a teacher and public lecturer. After teaching for 10 years at a Quaker boarding school in Kendal, he moved on to a teaching position in the burgeoning city of Manchester. There he joined the Manchester Literary and Philosophical Society, which provided him with a stimulating intellectual environment and laboratory facilities. The first paper he delivered before the society was on color blindness, which afflicted him and is sometimes still called Daltonism.
dalton1-profile.jpg
John Dalton, F.R.S., engraved by William Henry Worthington after an 1814 painting by William Allen, published June 25, 1823, in Manchester and London. Note the charts with Dalton’s atomic symbols lying on the table.
Theories of Atomism and the Law of Partial Pressures
Dalton arrived at his view of atomism by way of meteorology, in which he was seriously interested for a long period: he kept daily weather records from 1787 until his death, his first book was Meteorological Observations (1793), and he read a series of papers on meteorological topics before the Literary and Philosophical Society between 1799 and 1801.
The papers contained Dalton’s independent statement of Charles’s law (see Joseph Louis Gay-Lussac): “all elastic fluids expand the same quantity by heat.” He also clarified what he had pointed out in Meteorological Observations—that the air is not a vast chemical solvent as Antoine-Laurent Lavoisier and his followers had thought, but a mechanical system, where the pressure exerted by each gas in a mixture is independent of the pressure exerted by the other gases, and where the total pressure is the sum of the pressures of each gas. In explaining the law of partial pressures to skeptical chemists of the day—including Humphry Davy—Dalton claimed that the forces of repulsion thought to cause pressure acted only between atoms of the same kind and that the atoms in a mixture were indeed different in weight and “complexity.”
Arnold Thackray describes how John Dalton's book on meteorology led to his discovery of the nature of atoms.
Experiments on Atomic Weights and Structures
dalton2.jpg
Elements and their combinations as described in John Dalton’s New System of Chemical Philosophy (1808–1827).
He proceeded to calculate atomic weights from percentage compositions of compounds, using an arbitrary system to determine the likely atomic structure of each compound. If there are two elements that can combine, their combinations will occur in a set sequence. The first compound will have one atom of A and one of B; the next, one atom of A and two atoms of B; the next, two atoms of A and one of B; and so on. Hence, water is HO. Dalton also came to believe that the particles in different gases had different volumes and surrounds of caloric, thus explaining why a mixture of gases—as in the atmosphere—would not simply layer out but was kept in constant motion. Dalton consolidated his theories in his New System of Chemical Philosophy(1808–1827).
As a Quaker, Dalton led a modest existence, although he received many honors later in life. In Manchester more than 40,000 people marched in his funeral procession.
Visit the Science History Institute to learn more about Dalton.
The information contained in this biography was last updated on December 4, 2017.
John Dalton
Experiments with gases that first became possible at the turn of the nineteenth century led John Dalton in 1803 to propose a modern theory of the atom based on the following assumptions.
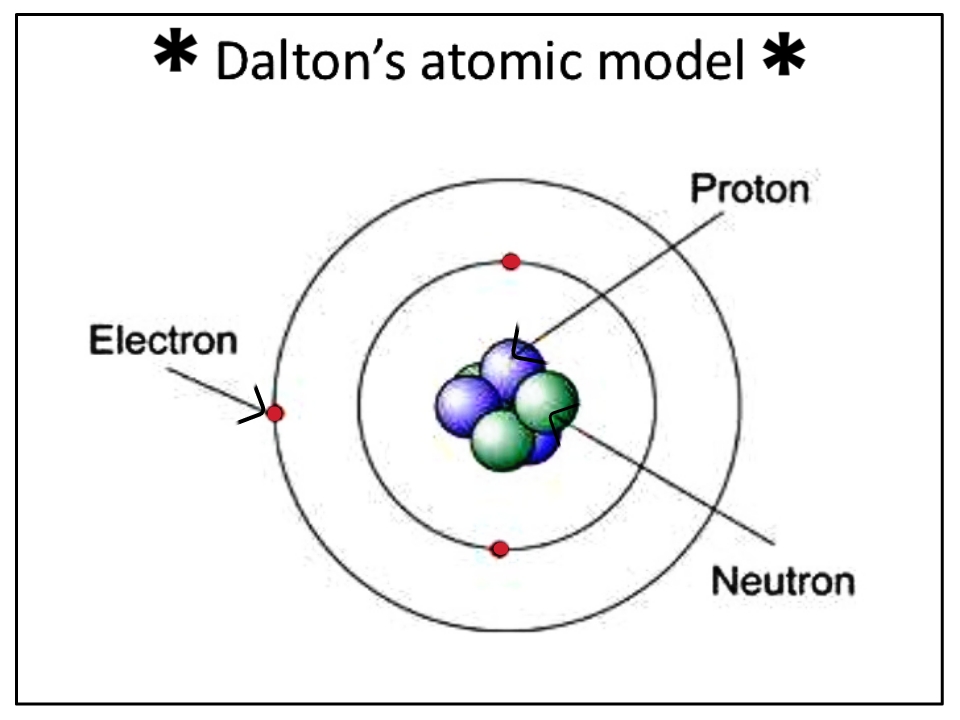
1. Matter is made up of atoms that are indivisible and indestructible.
Monopoly full version apk. 2. All atoms of an element are identical.
3. Atoms of different elements have different weights and different chemical properties.
4. Atoms of different elements combine in simple whole numbers to form compounds.
John Dalton Atom Date
5. Atoms cannot be created or destroyed. When a compound decomposes, the atoms are recovered unchanged.
John Dalton was the first to recognize that the total pressure of a mixture of gases is the sum of the contributions of the individual components of the mixture. Super smash flash 2 9 0b download. By convention, the part of the total pressure of a mixture that results from one component is called the partial pressure of that component. Dalton's law of partial pressures states that the total pressure of a mixture of gases is the sum of the partial pressures of the various components. Naruto to boruto shinobi striker walkthrough.

PT = P1 + P2 + P3 + ..
Dalton derived the law of partial pressures from his work on the amount of water vapor that could be absorbed by air at different temperatures. It is therefore fitting that this law is used most often to correct for the amount of water vapor picked up when a gas is collected by displacing water.
John Dalton was not familiar with Richter's work when he developed his atomic theory in 1803. By 1807, however, references to this work appeared in Dalton's notebooks, and Dalton's contemporaries viewed his atomic theory as a way of explaining why compounds combine in definite proportions.
John Dalton Atomic Model
Consider water, for example. In his famous textbook, Trait lmentaire de Chimie, which was published in 1789, Lavoisier reported that water was roughly 85% oxygen and 15% hydrogen by weight. Water therefore seemed to contain 5.6 times more oxygen by weight than hydrogen. Dalton assumed that water contains one atom of hydrogen and one atom of oxygen, as shown below, and concluded that an oxygen atom must weigh 5.6 times more than a hydrogen atom. On the basis of such reasoning, Dalton constructed a table of the relative atomic weights of a handful of elements.
John Dalton Atom Model Diagram
| Dalton assumed that water contains one atom of hydrogen and one atom of oxygen and concluded that the relative weight of the oxygen atom must be 5.6 times as large as the hydrogen atom. |
| History of Chemistry |
John Dalton Atomic Mass
| Experiments Index |
| Scientists Index |
Influence Of Atomic Theory
